Plates inside the AGM batteries are mostly the electrodes. The electrodes are found in the positive and negative formation. The plates inside the AGM battery are different from the other batteries. Mainly, lead plates are used as the electrodes in the AGM battery. Here the question arises how do AGM plates work?
The cell chambers of the AGM batteries have a sandwiched structure of electrode plates. A pure lead plate is used in the AGM battery. Very thin plates are used to make the sandwiched form. In between the cathode and anode, there is an AGM separator that absorbs the electrolytes. Current flows from the plates to and from the electrolytes.
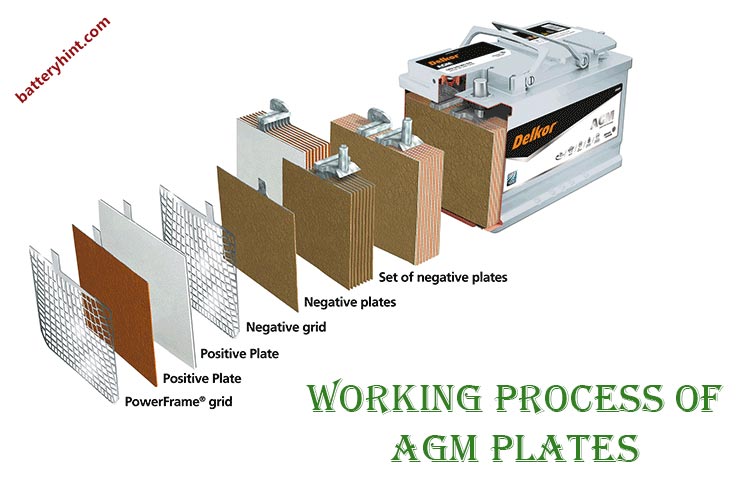
The working process of AGM plates
Very thin Lead plates are used in making the cells of the AGM battery. In order to understand the working principle of the AGM plates, the construction needs attention. This section thus shall answer the question of how to do AGM plates work.
Construction of battery cell
AGM batteries have a unique form of cell in a very compact and closed structure.
- Each cell of the battery has two pure lead plates.
- One of the plates is the positive electrode and one is the negative electrode.
- In between the lead plates, there is an AGM separator. It holds and absorbs the liquid electrolyte.
- The outermost side of the plates has grids.
- All these are highly compressed and closely packed to each other. It acts as a single cell with two terminals.
- In an AGM battery, there are six cells that are interconnected to each other in a series connection. All these combined produce power.
Working Principle
The AGM battery works on an electrochemical reaction basis. Chemical energy is converted to electric energy. Electrons from the positive electrode otherwise known as cathode go to the electrolyte. These electrons flow through the cell and are received by the negative electrode. This maneuver produces electricity and completes a series of oxidation-reduction reactions.
Are AGM plates different from other batteries?
The plates used in the AGM batteries are different from the plates of the other batteries. There are 4 major types of batteries used widely. These are the Gel-filled battery, Absorbed Glass Mat battery, Li-ion battery, and the standard lead-acid battery.
Comparison Between AGM plates and Gel-filled Battery plates
In the AGM battery, the plates are made in a very thin formation. Therefore, a higher volume of lead plates can be used to make the cells of the battery. Besides, the lead plates are placed very confined in closed-packed structures.
There is another form of AGM battery that has spiral cells and electrode plates. These Optima batteries have higher vibration resistivity and spill proofing than flare plates.
Plates of the GEl-filled battery receive electrons from the silica gel-based electrolytes. This process is more effective than the AGM battery. Here, electrons are transferred through the absorbed electrolytes to the glass mat.
The gel-filled batteries possess blocks of lead plates. In each cell chamber, the plates are placed in a flat position. Besides, impure Lead plates are used in the Gel-Filled batteries. Therefore, the effectiveness of gel-filled batteries is lower than AGM batteries.
Electrode plates of the Gel batteries are lesser than the AGM batteries. It is because of the thickness of the plates. In the gel batteries, the plates are positioned separately. There is a positional gap between the positive and negative electrodes. AGM battery cells have a very compact, closely packed structure. Here, the plates are sandwiched between the glass mat.
Comparison between AGM plates and Standard Battery plates
In the Standard lead-acid batteries, Lead-alloys are used as plates. Mostly Calcium or Magnesium are used to make the alloy plates. These are placed being dipped inside the liquid electrolyte. The structure of the plates is block-shaped with a relatively higher width. Whereas, in AGM batteries the very thin plates are used.
The amount of electrode plates in the standard battery is lower than the AGM battery. Moreover, the positioning of the plates is quite different. The cell chambers of the AGM battery act separately. Here, the plates, glass mat absorbing the electrolytes are compressed and attached to each other.
But for the Standard flooded battery, the electrodes are used as bars. The cell chambers are flooded with liquid electrolytes, and electron exchange takes place by direct contact between the electrolyte and the plates.
Comparison between AGM plates and Standard Battery plates
Finally, the Li-ion batteries have bars of different highly conductive metal than lead. These plates are more malleable than the lead plates. But Lithium-ion battery plates are more reactive than lead plates of the AGM batteries. Because of the higher position in the spectrochemical series.
That means it can easily lose free electrons in the electrolyte which causes the production of electricity. Secondly, in Lithium-ion batteries the formation of electrodes is very different from the AGM battery. There are no separate cells in the li-ion batteries like AGM batteries.
The entire Li-ion battery acts as a cell. Electrode plates used in the Li-ion batteries are mostly made of Copper and Aluminum; both of these are more conductive than lead plates. Besides, Copper and Aluminum are very light compared to the Lead plates.
In Li-ion batteries, copper and Aluminum bars are used as the anode and cathode respectively. But for the AGM battery, both cathode and anode role is done by the Lead plate. That is why the charge holding capacity and power delivery of the Li-ion battery is higher than the AGM battery.
Comparing the sustainability of the plates, the AGM battery lags far behind. LEad is very harmful to the environment and for animals as well. These are rated as toxic. But, Li-ion battery plates are non-toxic and relatively more friendly than Lead plates. Recycling the Li-ion plates is easier than AGM battery plates.
FAQ
This section reveals the answers to the questions asked by the consumers regarding AGM plates.
Do AGM batteries have plates?
AGM batteries have Lead plates. These act as the positive and negative electrodes inside the cell. There is an AGM separator in between the two plates which absorbs the electrolytes. The plates of the AGM batteries are very thin and made of pure Lead.
What is an AGM flat plate battery?
There are mainly two types of AGM batteries available in the marketplace. The flat plate AGM batteries have to flare thin confined cells. On the other hand, spiral AGM batteries have the cylindrical shapes of electrode plates made of lead as well.
Conclusion
Plates in the AGM batteries work as the electrodes required for carrying out the electrochemical reaction. For different batteries, the plates act differently with different effectiveness. Even the material of the plates differs from each other.
If you are planning to repair or modify your AGM battery. Then it is imperative for you to know how do AGM plates work. Because without knowing that you can not buy the required amount of tools and accessories.
Moreover, for regular maintenance purposes, equalization, etc the working principle of the plates in the AGM batteries should be known clearly. Because the plates in the different batteries work differently due to the material and conductive nature.